
Research on Retraining movement
-Biofeedback and Bayesian Analysis of Neurophysiological Signals
Failure of Motor Learning
Improvement of performance with practice is a fundamental assumption about many motor skills. However, a child with a movement disorder makes repeated attempts at movement every day yet does not improve performance. This has led to Dr. Sanger’s theory of “failure of motor learning.” This theory describes conditions under which practice of a movement does not lead to the best possible performance. It is directly relevant to children with movement disorders, since their movements do not improve despite constant attempts both in activities of daily living and in rehabilitation therapy. Failure can occur due to (1) sensory limitations that decrease perception of movement errors, (2) lack of experience of approximately successful performance, (3) computational limitations on the learning or movement control system, and (4) biomechanical constraints on movement. In all cases, the child has the potential ability to perform better, yet they cannot achieve this potential through practice alone.
Theoretical analysis of neural network models used for robotic adaptive control shows that type 1 and type 2 failure can occur even in healthy motor systems. In type 1 failure of motor learning, there is a deficit of sensory processing or an absence of appropriate attention such that the child does not respond to movement errors. In type 2 failure of motor learning, there is a deficit of motor performance such that the child has no examples of successful movement and thus practices (and solidifies) only the incorrect movement. Extensions of these two failure modes capture many of the ways in which an adaptive controller may fail. We have demonstrated that both types of failure can occur in control subjects and prevent learning a multiple-muscle pattern of movement. We have also demonstrated in a small number of children with dystonia that failure of motor learning may be responsible for abnormal muscle contraction in certain tasks. Interestingly, confusion of sensory information about muscle patterns can induce abnormal movements in control subjects, suggesting the possibility of a sensory deficit as a cause of abnormal movements in dystonia. Provision of adequate sensory information or guiding to a single successful example of performance can be used to circumvent failure of motor learning and improve control of muscle groups. Further research is needed to determine how to provide appropriate sensory information or guidance for general categories of abnormal movements.
Biofeedback and Bayesian Analysis of Neurophysiological Signals
Neurophysiological signals such as the surface electromyogram, scalp potentials, or multiple-electrode extracellular recordings are considered to be “noisy” or random and only amenable to interpretation following aggressive smoothing or averaging. However, such smoothing or averaging removes rapid temporal structure so that the resulting signals do not accurately reflect the precise and rapid movements that are possible. Dr. Sanger has developed a set of techniques based on stochastic differential equation models and Bayesian nonlinear signal processing that permit accurate and rapid on-line interpretation of neurophysiological signals.
In order to use these techniques for motor retraining, we have constructed prototypes of a special-purpose electromyography electrode that incorporates a fast microcontroller so that voluntary motor drive can be estimated in real-time and used to control feedback to the subject and assistive communication devices (see figure below). One previous difficulty with the use of EMG for control is that it may not be apparent to a child which muscle is providing the control. For example, a child with dystonia may simultaneously contract many muscles in their arm, and it may be difficult to determine which muscle is controlling an EMG switch. This is particularly problematic if the goal of the EMG device is not just immediate control, but also teaching the child how to activate specific muscles. Therefore we have developed hardware and software for a vibrating EMG electrode. The electrode is constructed so that it vibrates with a strength that is proportional to the degree of muscle activation. Thus a child can immediately feel the electrode that is active, and the feeling occurs at the responsible muscle. Vibrating motors were selected with an oscillation frequency that will give a strong sensation in the muscle, and this may mimic or enhance the normal sensation of movement.
The vibrating EMG electrodes solve another important problem with biofeedback. Essentially all current biofeedback systems that are used for muscle training use sound as the feedback. In addition to the problem that sound does not indicate the responsible muscle, it also is disturbing both to the children and to anyone near them. Thus devices cannot be worn during school, and children with a high startle response may startle every time the device activates. The EMG device is very quiet and unobtrusive and can be worn during school. It does not seem to provoke a startle response. The current version includes a Bluetooth™ interface so that it can also be used as a switch to control a computer for children who do not have accurate use of their hands. Pilot testing using these electrodes for biofeedback in two children with severe dystonia has shown potential efficacy for retraining abnormal muscle patterns and learning new muscle patterns.
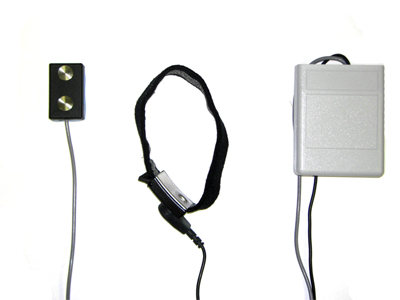 Prototype of the EMG biofeedback device. The electrode with vibration motor, microcontroller, variable-gain amplifier, and motor control electronics is at the left. The electrode holding strap with ground contact is in the middle. The battery and Bluetooth interface is in the belt pack at the right. The electrode including analog electronics and microcontroller circuitry is only 1.5 inches long.
Prototype of the EMG biofeedback device. The electrode with vibration motor, microcontroller, variable-gain amplifier, and motor control electronics is at the left. The electrode holding strap with ground contact is in the middle. The battery and Bluetooth interface is in the belt pack at the right. The electrode including analog electronics and microcontroller circuitry is only 1.5 inches long.
Dr. Sanger has demonstrated that these techniques can be applied to multiple-electrode recordings to extract a single signal that is “represented” by a population of neurons. We have extended these techniques to estimate the intended movement based on an inaccurate or variable movement made by a child. This extension has facilitated the design of a gesture-recognition joystick that learns the specific patterns of movement of an individual child and then compensates for inaccuracy in movement to estimate the underlying intent. Bayesian signal processing has potentially widespread applicability that has been limited by its intense computational requirements. By demonstrating efficient algorithms for real-time implementation, our work for the first time permits application of these methods for processing of biological signals.
Medication Trials
Over the past six years we have completed several clinical trials. We have shown that botulinum toxin injected into the biceps of children with arm dystonia increases the speed of reaching. The results suggest that abnormal biceps activation occurs during voluntary movement and may interfere with movement and motor learning. This was a dose-escalation trial and provides some of the first dosing guidelines for the use of botulinum toxin type B in children. It also suggests that testing with the robotic arm will reveal that biceps activity contributes not only to passive stiffness but also to impaired active reaching.
Dr. Sanger directed a multi-center open-label trial of trihexyphenidyl in children with secondary arm dystonia. Trihexyphenidyl probably affects learning and plasticity within both the cortex and basal ganglia and thus an effect on dystonia could be due to modification of learning and adaptation mechanisms. This study was performed as the initial trial of the Child Motor Study Group (CMSG) that Dr. Sanger founded and continues to co-direct. The results of the clinical trial showed efficacy, but more importantly demonstrated the unexpected finding that children with hyperkinetic features may worsen during treatment. This suggests that such children should be excluded from further trials, and it provides further evidence that hyperkinetic dystonia is distinct from hypertonic dystonia. Retrospective studies from other clinics suggest that trihexyphenidyl’s efficacy is much greater in younger children. Treatment of children below two years of age can lead to very rapid and long-lasting elimination of dystonic symptoms. It is interesting to hypothesize that the mechanism of persistent improvement could be due to the effect of trihexyphenidyl on learning. We are currently working with other members of the CMSG to design a randomized trial of trihexyphenidyl in young children.
We recently completed a trial of short-term oral baclofen in children with ankle spasticity. This was a departure from the usual intent to study arm movements, but was based upon a theoretical conjecture about plasticity in corticospinal systems. In particular, the goal was to provide preliminary support for the hypothesis that spasticity may prevent increases in voluntary strength by reducing the need for voluntary activation of antigravity muscles. The study showed that voluntary ankle muscle activity increased following one month of treatment with oral baclofen. This finding is consistent with the hypothesis that the presence of spasticity during early development could prevent the development or recovery of voluntary control of the corticospinal system. Therefore early and aggressive treatment with anti-spasticity agents could change the developmental course and help facilitate long-lasting recovery of corticospinal control.